When beginning to understand what people are thinking, there are few places better to start than the brain. There are several methods that allow different approaches and insights to detect changes in brain activity, but none are as direct as EEG (1).
By detecting the changes in electrical activity that occurs in the communication of neurons, the signal collected by EEG essentially reflects a direct translation of neural activity into data (2).
While EEG is not a brain imaging method with the same high spatial resolution as MRI, the temporal resolution is effectively unparalleled. Combine all of this with its relative portability to MRI, PET, etc., and it’s clear why EEG is one of the main methods used in neuroscience and other fields today.
This neuroimaging method therefore presents a great opportunity for researchers looking to understand more about the brain, but as with any neuroimaging method, there are challenges present in the setup, and execution of studies using this device.
In an effort to help with this, we have detailed five key points below that should be taken into account before setting up an EEG experiment. There may also be other aspects to consider in your own experiment, so we’d always recommend seeking out expert help and guidance where possible. When you become an iMotions customer you will have access to our team of experts to help you every step of the way.
5 Essentials for Optimal EEG Equipment Set up :
- EEG Equipment
- How to place your EEG on Participant
- EEG Electrical Conduction
- Minimize Movement for EEG data collection
- What does EEG Data look like?
1. EEG Equipment

Check out: What is EEG (Electroencephalography
The first step in launching into research using EEG is deciding which device to purchase. There are several factors that will come into play, depending on the type of research that you are doing.
Channels
Perhaps the first thing to consider with the device is the number of channels you require. More channels means more data – often a good thing, but not an essential in all cases. If you’re only interested in brain activity from the frontal regions of the brain, you don’t necessarily need a 128-channel system.
Sampling Rate
The Nyquist theorem (not as scary as it sounds) states that for any signal you want to uncover, you’ll need a sampling rate twice as fast. In order to uncover the fastest signals of interest with EEG, a sampling rate of 128 Hz comfortably covers this (3). A faster sampling rate of course generates more detailed data, so if your budget allows, you’ll want to go for more rather than less.
Amplifier
Often the most expensive part of the EEG equipment, the amplifier (not too surprisingly) amplifies the signal recorded from the electrodes, making the data essentially more visible. This is one of the most critical aspects of the EEG equipment for assuring data quality, so is not something to ignore (some headsets don’t come with an amplifier, so the signal later has to be amplified).
2. How to place your EEG on Participant
Ensure that the placement of the electrodes is correct. This involves both the electrodes which you collect data from, and the reference electrode(s) that help baseline the data collection.
For ensuring that the electrodes are placed in the right position, it’s important that the central electrodes align with set positions on the scalp (e.g. the electrode in position “Cz” is in the center). Most electrode positioning is done in accordance with the 10-20 system, a standardized map for EEG recordings (4, 5).
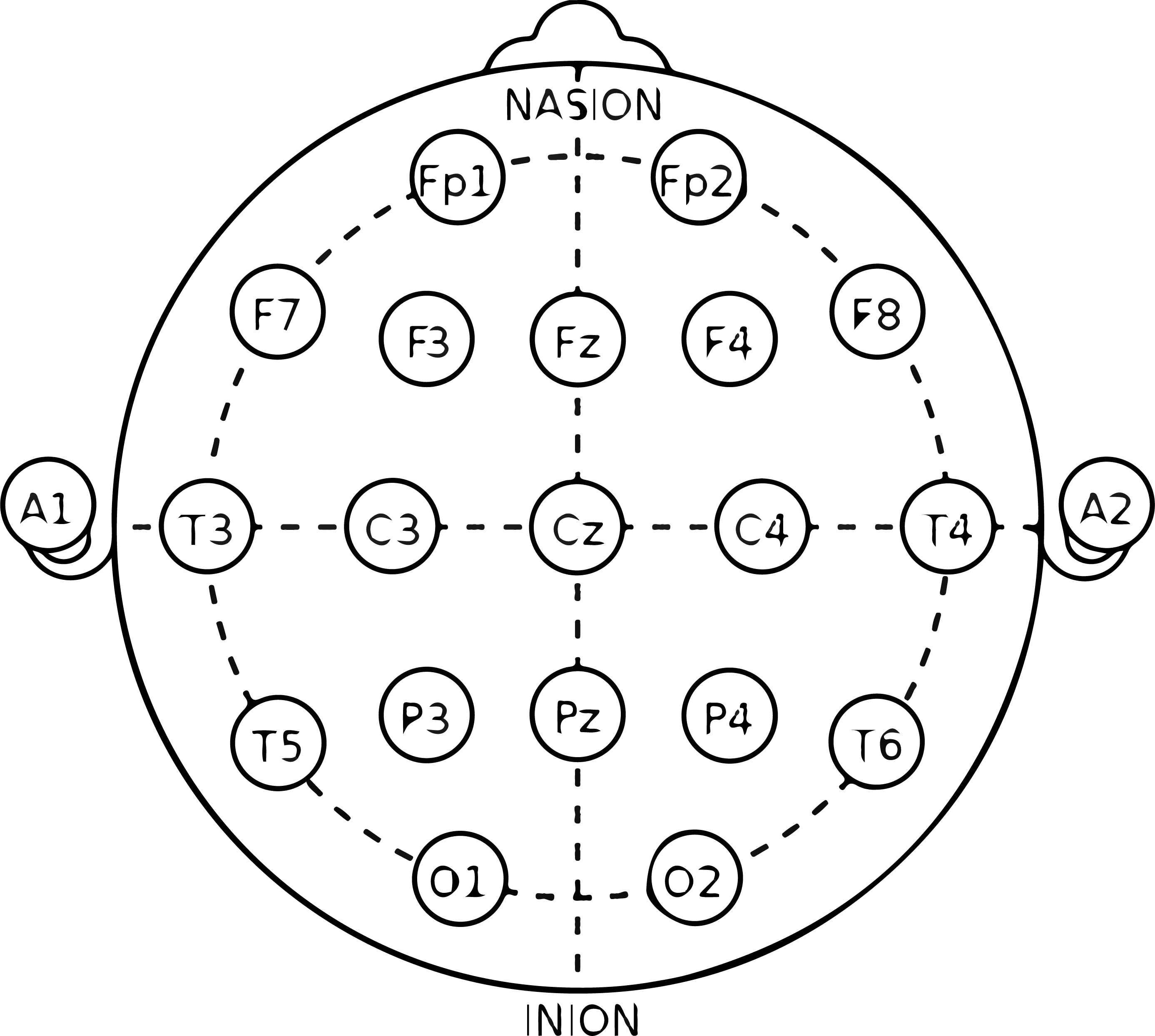
If the EEG system you are using features an EEG cap, then it’s a simple matter of placing the electrodes within the corresponding positions in the cap, and checking the positions are correct (some systems, like the ABM B-Alert headsets, make this easier by fixing the electrodes in position together).
3. EEG Electrical Conduction
In order to detect the electrical charge generated by the brain, the signal has to go through the scalp and to the electrode. To maximize the amount of signal detected, it’s best to help conduct the electricity as much as possible.
In the context of an EEG headset, this means ensuring that the scalp is clean to reduce impedance (it’s unlikely you’ll need to send your participant to go shower, but applying alcohol wipes to areas that will have contact with electrodes can improve the electrical conduction quality).
Electrical conduction is also always improved with the application of conduction gel (to a limit), so adding this in-between the electrode and scalp is recommended. Dry and semi-dry electrodes are also available, and can offer a quicker solution to collecting data. While they typically may not offer as much signal fidelity, it will depend on your research question where you fall on the divide between the time required for setup and data quality.
4. Minimize Movement for EEG data collection
Movement from a participant wearing an EEG headset is never a good thing for the data – any slippage or disturbance to the electrode can cause some kind of artifact or inaccuracy. While you don’t want the headset to move around on the head of the participant, it might be that your experiment requires the participant to get up and about.

There are ways of handling this kind of experiment – simply choose a device that allows mobile recording. Devices from NeuroElectrics, ABM, and Emotiv (among others) all offer EEG headsets that are designed for mobile use (often through Bluetooth, but also WiFi connections).
While keeping a stable and controlled environment is ideal for maximal data quality, general movement is tolerated by these devices, allowing you to introduce more ecological validity into your experiments.
5. What does EEG Data look like?
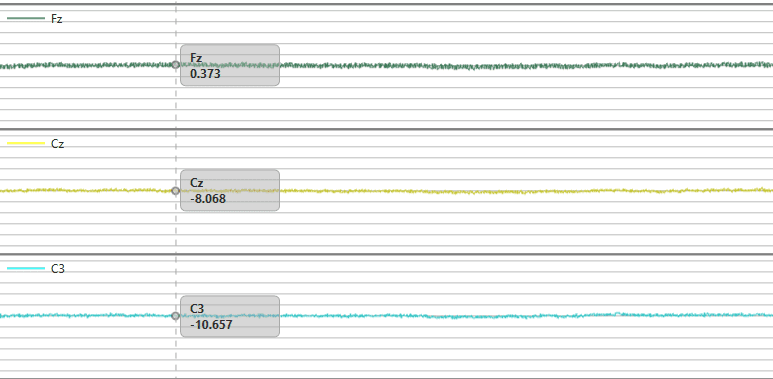
As the old maxim goes “Garbage In, Garbage Out” – the same goes for data. Ideally your experimental conditions, setup, and equipment will all convene to ensure as high quality data collection as possible, and the process will be easier from there.
There are however things to keep in mind both before and after the experiment to help you get the most accurate findings for your study.
Learn more: Everything you can do with the iMotions EEG Module
Consider frontal asymmetry – a widely used measure that can provide values associated with feelings of approach or avoidance. Reliable analysis of this data is dependent on a long enough data collection period, of at least 30 seconds (6). It goes without saying that you must ensure that your experimental design allows for this if you want to collect this metric.
Accurate data collection from other devices may be dependent on a proper calibration period which should be carried out. Following data collection, further work with the data will likely be required, such as with removing artifacts or even entire recordings if needed (7).
Conclusion
It’s worth keeping in mind that while EEG experiments can offer great insight into brain activity and cognitive processes, the execution of these studies is reliant on proper experiment setup and execution.
Your exact research question and needs will of course determine what other steps you may need to take (these 5 essentials are only really the beginning), but if you follow these tips then you are well on your way to carrying out reliable research.
Free 59-page EEG Guide
For Beginners and Intermediates
- Get a thorough understanding of the essentials
- Valuable EEG research insight
- Learn how to take your research to the next level

References
1. Jackson AF, Bolger DJ. (2014). The neurophysiological bases of EEG and EEG measurement: a review for the rest of us. Psychophysiology, 51:1061–71.10.1111/psyp.12283
2. Buzsáki G., Anastassiou C. A., Koch C. (2012). The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420. 10.1038/nrn3241
3. Weiergraber M, Papazoglou A, Broich K, Muller R. (2016). Sampling rate, signal bandwidth and related pitfalls in EEG analysis. J Neurosci Methods, 268:53–5.
4. Jasper, Herbert H. (1958). Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology. 10 (2): 370–375.
5. Niedermeyer, E., & Lopes da Silva, F. H. (1999). Electroencephalography: Basic principles, clinical applications and related fields. Baltimore, MD: Williams & Wilkins.
6. Coan, J. A., & Allen, J. J. B. (2002). The Asymmetrical Brain. Richard J. Davidson and Kenneth Hugdahl (eds.). Boston, MA, USA; MIT Press.
7. Urigüen JA, Garcia-Zapirain B. (2015). EEG artifact removal-state-of-the-art and guidelines. J Neural Eng, 12: 31001