Advancing the understanding of pain measurement through multimodal assessment is crucial in accurately evaluating pain experiences. Through comprehensive and diverse approaches to pain measurement, researchers aim to enhance diagnostic precision and treatment effectiveness. This study delves into the intricate methods and tools for measuring pain, shedding light on the complexity of pain assessment.
Table of Contents
Pain is a complex, subjective experience-rooted in neurobiology and shaped profoundly by perception, emotion, cultural context, and cognitive interpretation. While patient self-report remains the cornerstone of assessing pain, it is inherently constrained by language, memory, and individual expressiveness.
It is unlikely that an objective measure of pain will ever emerge, given that the experience of pain is fundamentally a personal one. A stimulus that is mildly uncomfortable to one individual may be profoundly debilitating to another, illustrating the wide interindividual variability in how pain is perceived, appraised, and expressed.

However, continuous advancements in multimodal biosensing have opened the door to new avenues of pain assessment that integrate physiological, behavioral, and neurological signals to infer the presence and magnitude of pain.
This article explores the research applications of objective pain measurement using iMotions, a software platform designed to synchronize and analyze multimodal data across biosensors.
The Ontological Problem of Pain
Pain is not a single signal but a complex emergent state. It may arise in some cases from nociception (the detection of harmful stimuli) but involves higher-order processing that transforms this signal into a conscious, emotionally laden experience. Neuroscientific research shows that pain is not confined to a “pain center” but distributed across a dynamic pain connectome, including the anterior cingulate cortex (ACC), insula, thalamus, somatosensory cortex, and prefrontal cortex.
This distributed nature makes it difficult to isolate pain in a purely biological sense. According to the International Association of Pain, pain may exist with actual or potential tissue damage (nociceptive pain), with nerve damage (neuropathic pain), or even in the absence of clear evidence of tissue/nerve damage (nociplastic pain).
Any attempt to measure pain, then, must account for multi-dimensionality – including physiological arousal, affective expression, cortical activity, and behavioral output.
The Case for Multimodal Pain Measurement
To approach pain as a near-measurable phenomenon, we must triangulate across multiple modalities. The iMotions platform offers synchronized integration of several biosensors, each capturing distinct dimensions of the pain experience:
| Modality | Measures | Role in Pain Detection |
| Facial Expression (Affectiva) | Action Units (FACS) | Detects involuntary expressions linked to pain |
| Electrodermal Activity (EDA) | Skin conductance | Reflects sympathetic arousal due to pain |
| Heart Rate & Heart Rave Variability (HRV) | Cardiovascular reactivity | Reflects pain-related modulation of time-based and frequency-based heart rate variability |
| Electromyography (EMG) | Muscle activation | Quantifies muscle strength (usually reduced when an affected limb or body part is under pain), muscle tension |
| Eye Tracking | Pupil dilation, fixations | Infers arousal and cognitive load from pain |
| Electroencephalography (EEG) | Cortical activity | Captures cognitive and sensory pain processing |
| Functional near-infrared spectroscopy (fNIRS) | Hemodynamic responses | Indirect changes of cortical activity |
iMotions automatically synchronizes these streams, offering researchers a composite, time-resolved view of how pain manifests across the body and brain.
Facial Expression Analysis: Reading Pain in the Face
Perhaps the most intuitive indicator of pain is the human face. Facial expressions of pain are evolutionarily conserved and can be detected even in infants and non-verbal individuals. Using FACS (Facial Action Coding System), specific Action Units (AUs) such as brow lowering (AU4), orbital tightening (AU6/7), nose wrinkling (AU9), and upper lip raise (AU10) have been associated with acute pain episodes.
iMotions, via integration with Affectivas AFFDEX software, provides real-time, automatic AU detection. Unlike manual FACS coding, which is time-intensive, this allows scalable analysis across hours of video, offering quantification of:
- Frequency of pain-related AUs
- Duration of expressions
- Co-occurrence patterns (e.g., brow + eyelid regions during needle insertion)
This data can be correlated with stimulus events and physiological changes to validate pain responses.
Electrodermal Activity (EDA): Capturing the Body’s Stress Signal
EDA is a measure of skin conductance modulated by eccrine sweat gland activity, which in turn is controlled by the sympathetic nervous system. Pain stimuli-particularly acute, unexpected ones-trigger phasic skin conductance responses (SCRs).
In iMotions, EDA data from sensors like Shimmer, BIOPAC and Plux, is visualized alongside stimulus events, allowing researchers to:
- Analyze peak amplitudes and latency post-stimulus
- Visualize tonic and phasic components
- Map habituation to repeated pain exposure
EDA is especially valuable in experimental paradigms involving controlled pain induction (e.g., cold pressor test, electrical stimuli) where time-locking is critical.
Neural Signatures of Pain
Electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) provide a non-invasive window into the brain’s processing of painful stimuli.
EEG:
Electroencephalography (EEG) is a widely used method to measure brain activity in both experimental and clinical pain settings. It records voltage fluctuations directly from the scalp and is employed to investigate neural responses, helping to identify brain patterns associated with various aspects of pain e.g. sensory processing, salience, attention, cognition, the effects of analgesic treatments, among others.
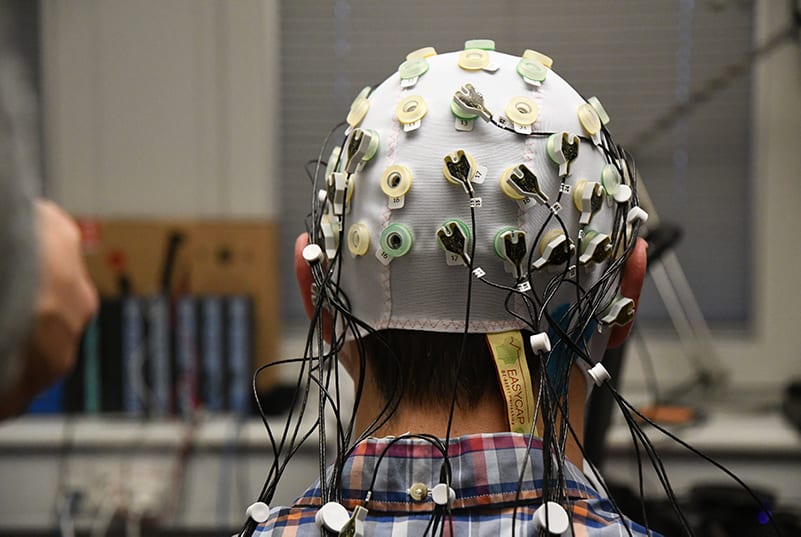
When doing resting-state EEG analysis, alpha suppression has been linked to stimulus intensity on the sensorimotor cortex and salience levels in operculoinsular areas. Facilitated theta oscillations have been reported in prefrontal and anterior cingulate cortices in patients with ongoing pain, as opposed to healthy pain-free controls, possibly attributed to the cognitive aspects of pain and endogenous pain inhibition.
Gamma-band synchronization has been historically linked to perceived pain intensity, but controversial findings warrant further research through highly controlled experiments, in order to circumvent the impact of motion-related confounding factors.
iMotions supports integration with various EEG systems, namely from Brain Products, Neuroelectrics Enobio, ABM B-Alert, and generally any EEG device supporting Lab Streaming Layer, allowing:
- Power Spectral Density (delta, theta, alpha, beta, gamma): analysis of cortical oscillations during pain
- Longitudinal tracking of neuroplastic changes in chronic pain patients
Recent studies using EEG with iMotions have begun to explore predictive models of subjective pain ratings, opening the door to AI-assisted diagnosis.
Some publications with iMotions
Alpha oscillations in chronic pain patients with iMotions
fNIRS:
fNIRS is a technique that measures brain function by monitoring shifts in oxyhemoglobin, which serves as an indirect indicator of cortical activity. In contrast to functional magnetic resonance imaging (fMRI), fNIRS offers advantages in terms of portability, cost-effectiveness, and the ability to allow subjects more natural movement.
A meta-analysis, which examined 13 different studies, provides compelling evidence regarding the brain’s response to painful stimuli in both chronic pain patients and healthy controls. The analysis revealed a significant increase in oxyhemoglobin levels in both the prefrontal and sensorimotor cortices when individuals experienced pain; but notably, the effect was more pronounced in the sensorimotor cortex.
The meta-analysis revealed distinct hemodynamic changes to noxious stimuli in individuals with chronic pain compared to healthy control groups. These findings collectively confirm the effectiveness of fNIRS as a tool for measuring how pain impacts these specific brain regions.
iMotions links for EEG and fNIRS material
EEG vs fNIRS: https://imotions.com/blog/learning/research-fundamentals/fnirs-vs-eeg/
EMG and HR/HRV: Somatic and Autonomic Correlates
Pain often reduces muscle strength on the affected body part as well as induces muscle tension, particularly in the corrugator supercilii (frowning) or trapezius (neck tension). iMotions computes various EMG analysis to detect how many and how often muscle contractions are done, among other metrics.
Heart rate (HR) and heart rate variability (HRV) further enrich the dataset. HR tends to increase during pain, while HRV-especially high-frequency components-tends to decrease, reflecting vagal withdrawal.
With iMotions, researchers can visualize:
Recovery curves post-intervention (e.g., analgesia)
Eye tracking
Pupillometry
Pupillometry, the measurement of pupil diameter, is a research tool in both experimental and clinical pain settings for assessing pain-related arousal due to its direct link with autonomic nervous system activity, particularly sympathetic activation. When individuals experience pain, the resulting physiological stress response triggers sympathetic outflow, leading to an increase in pupil dilation.
This allows researchers to gauge the intensity of the aversive emotional and physiological response to noxious stimuli, even in the absence of explicit verbal reporting such as in populations where verbal communication is limited (infants, critically ill patients, or individuals with cognitive impairments).
Pupil diameter is influenced by numerous factors beyond pain including luminance conditions (variations in luminance can directly influence pupil size, independent of arousal, making controlled and consistent lighting essential), medicine and coffee intake (various medications such as opioids, alcohol, certain antidepressants can directly affect pupil size), visual accommodation (changes in gaze or focus between near and far objects can cause pupils to constrict or dilate), and individual differences (the magnitude of pupillary response to pain can also vary significantly between individuals, making it challenging to establish universal thresholds for pain intensity).
Therefore, while promising, pupillometry often requires integration with other physiological or behavioral measures for a comprehensive understanding of pain intensity.
Fixation/saccadic metrics
Exploring how pain affects attention and information processing can be achieved through screen-based eye trackers as well as eye tracking glasses. Using screen-based eye trackers, a study published in the Journal of Pain showed that noxious stimulation elicits a reorientation of attentional resources, as indexed through a shift to less saccadic movements and longer fixations towards the pain source. The assessment of eye movements appears to offer a valuable supplementary methodology for exploring pain-related alterations in attention and how this can contribute to the modulation of pain.
A systematic review of 24 publications shows that the effect size on attentional bias towards predictive cues for pain is much larger than that of pain-related words and pictures. These results shed a light into creating relevant pain-related eye tracking studies.
iMotions offers both standard and advanced survey tools to create and log the answers to all kinds of affective, cognitive and pain catastrophizing questionnaires, Visual Analogue Scales for pain intensity assessments, among others through the new iMotions 10 study builder
Toward a Composite Pain Index
The future of pain detection lies not in choosing a single biosensor, but in constructing a multimodal pain index-a machine-learning-driven output that weights and integrates:
- Facial expression
- Autonomic signals (EDA, HR)
- EEG patterns
- Behavioral correlates
iMotions supports data export for statistical and AI modeling. This allows researchers to develop customized models tailored to their populations and research questions.
Example: A recent study trained a random forest classifier on synchronized EDA, facial AUs, and EEG features to predict self-reported pain scores with over 80% accuracy. Read about that here.
iMotions API allows real-time synchronization of external signals and streaming of raw unprocessed data to external engines (e.g., Python, R, MATLAB), if desired.
Free 44-page Experimental Design Guide
For Beginners and Intermediates
- Introduction to experimental methods
- Respondent management with groups and populations
- How to set up stimulus selection and arrangement